8 Is Enough
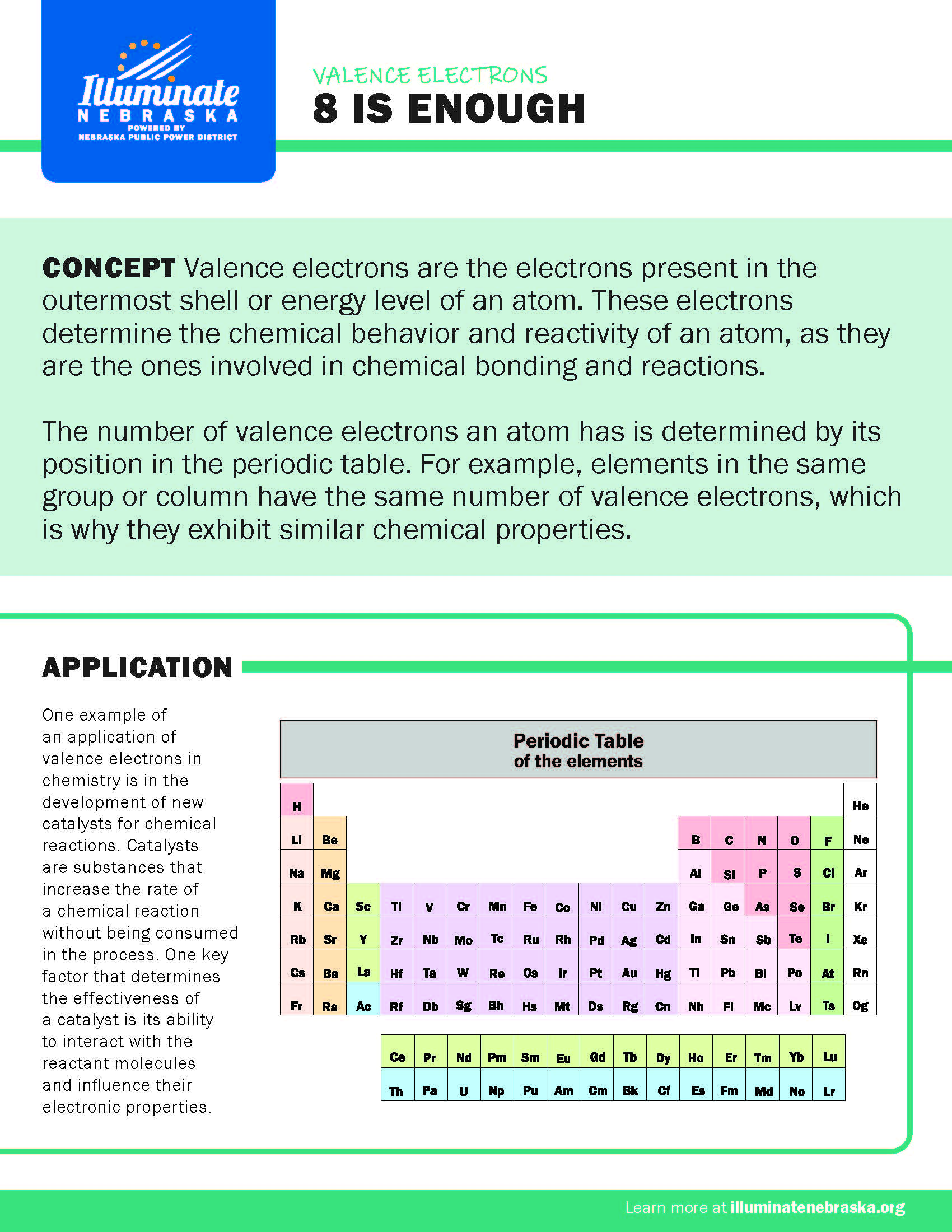
Valence electrons are the electrons present in the outermost shell or energy level of an atom. These electrons determine the chemical behavior and reactivity of an atom, as they are the ones involved in chemical bonding and reactions.
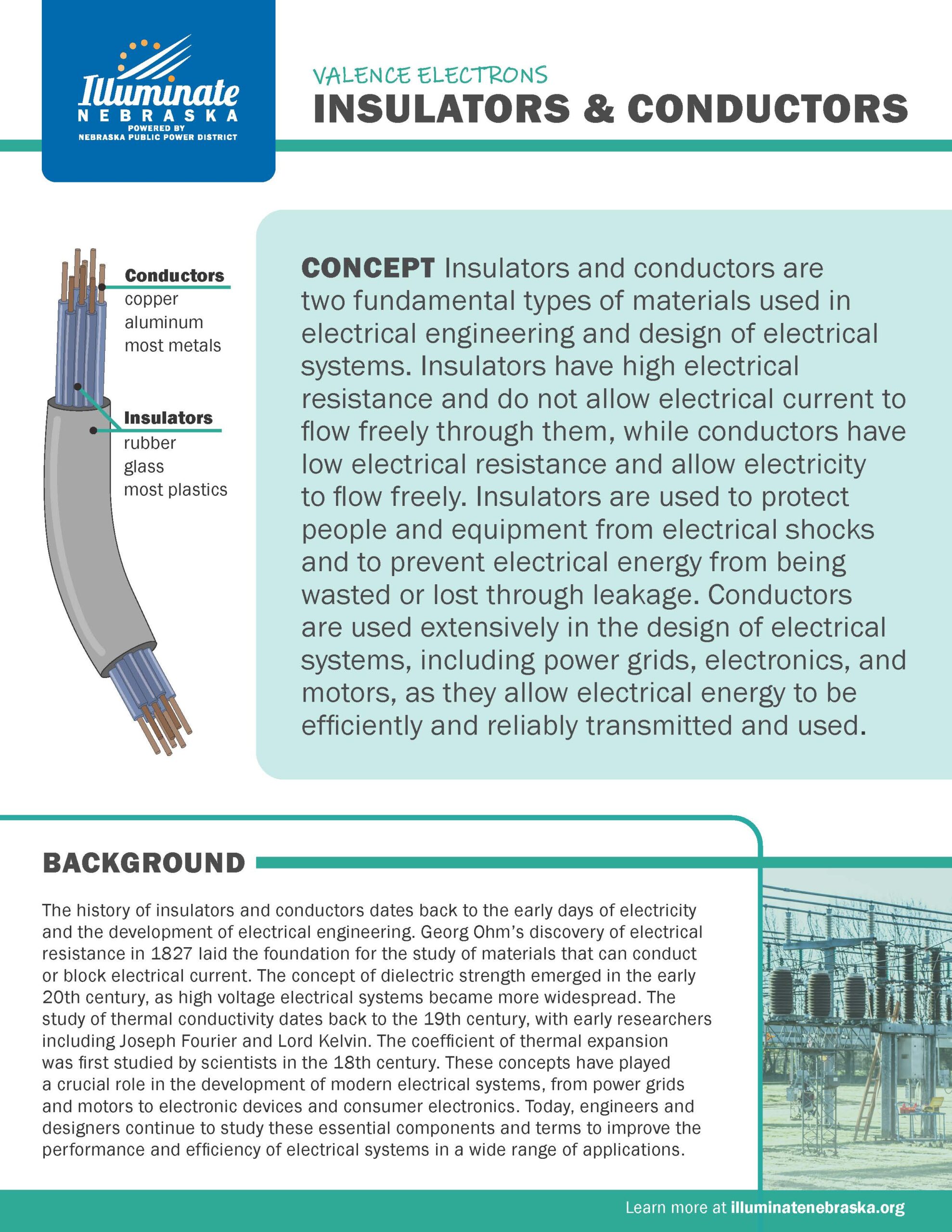
Insulators & Conductors
Insulators and conductors are two fundamental types of materials used in electrical engineering and design of electrical systems. Insulators have high electrical resistance and do not allow electrical current to flow freely through them, while conductors have low electrical resistance and allow electricity to flow freely.
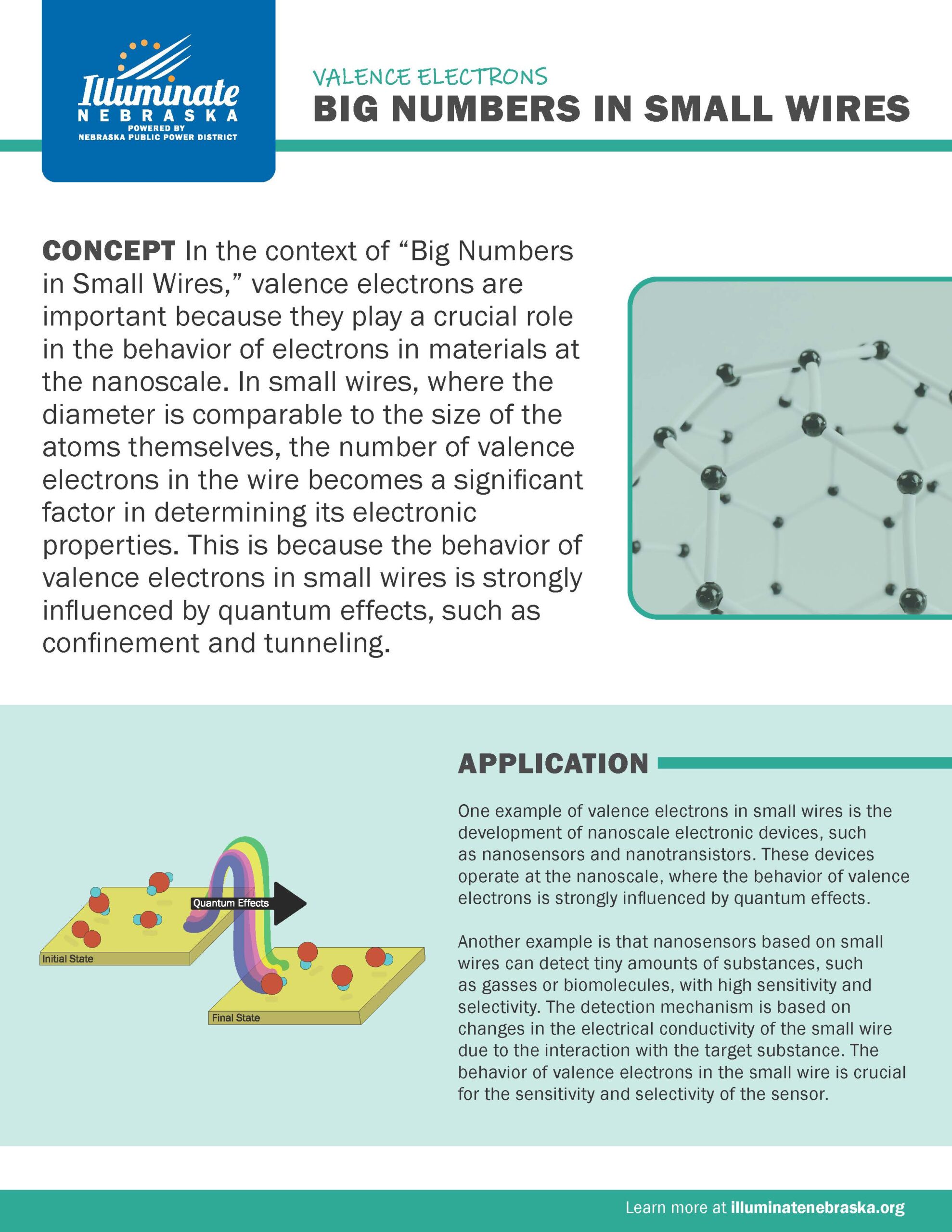
Big Numbers in Small Wires
In the context of “Big Numbers in Small Wires,” valence electrons are important because they play a crucial role in the behavior of electrons in materials at the nanoscale. In small wires, where the diameter is comparable to the size of the atoms themselves, the number of valence electrons in the wire becomes a significant factor in determining its electronic properties.
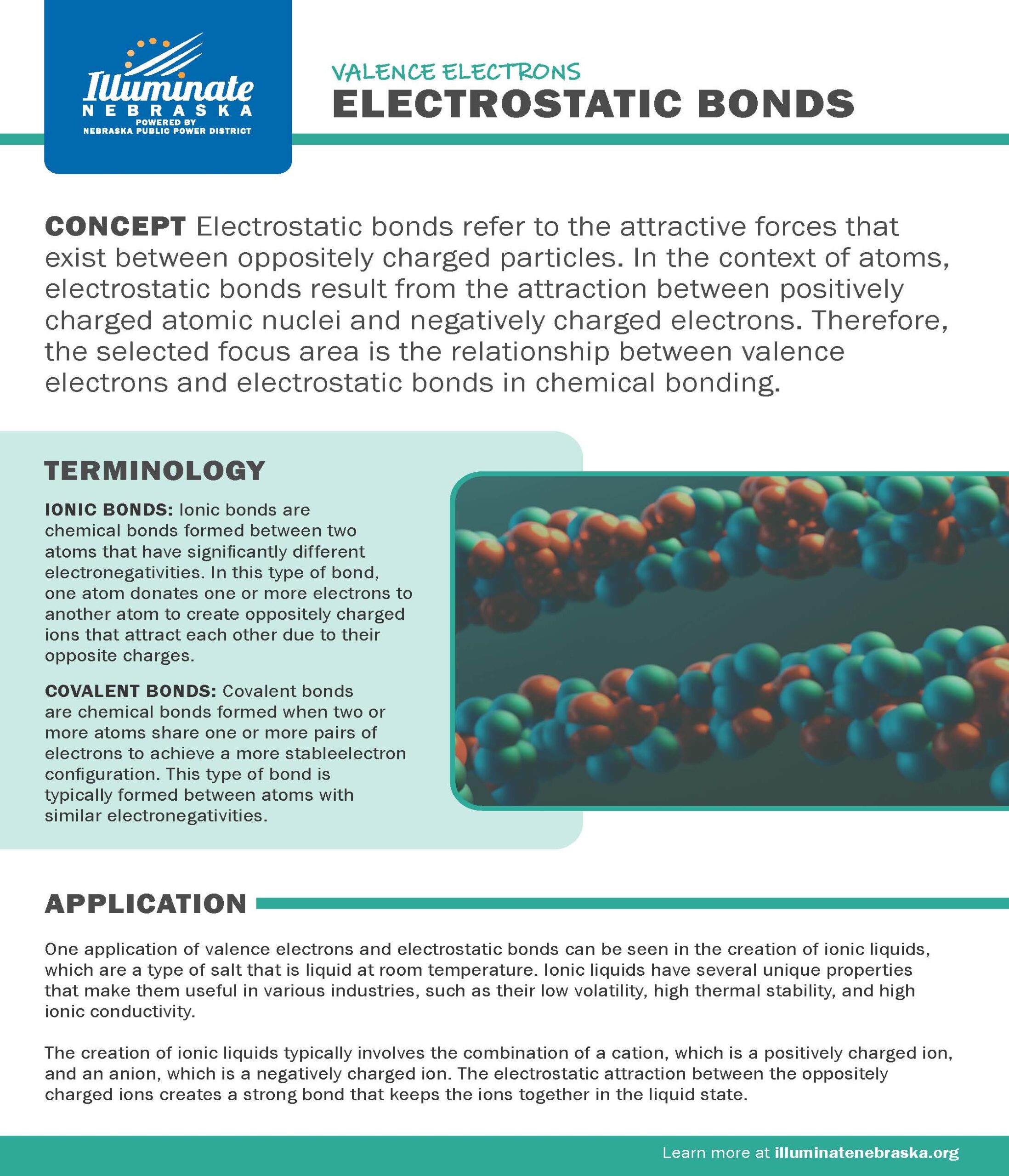
Electrostatic Bonds
Electrostatic bonds refer to the attractive forces that exist between oppositely charged particles. In the context of atoms, electrostatic bonds result from the attraction between positively charged atomic nuclei and negatively charged electrons. Therefore, the selected focus area is the relationship between valence electrons and electrostatic bonds in chemical bonding.
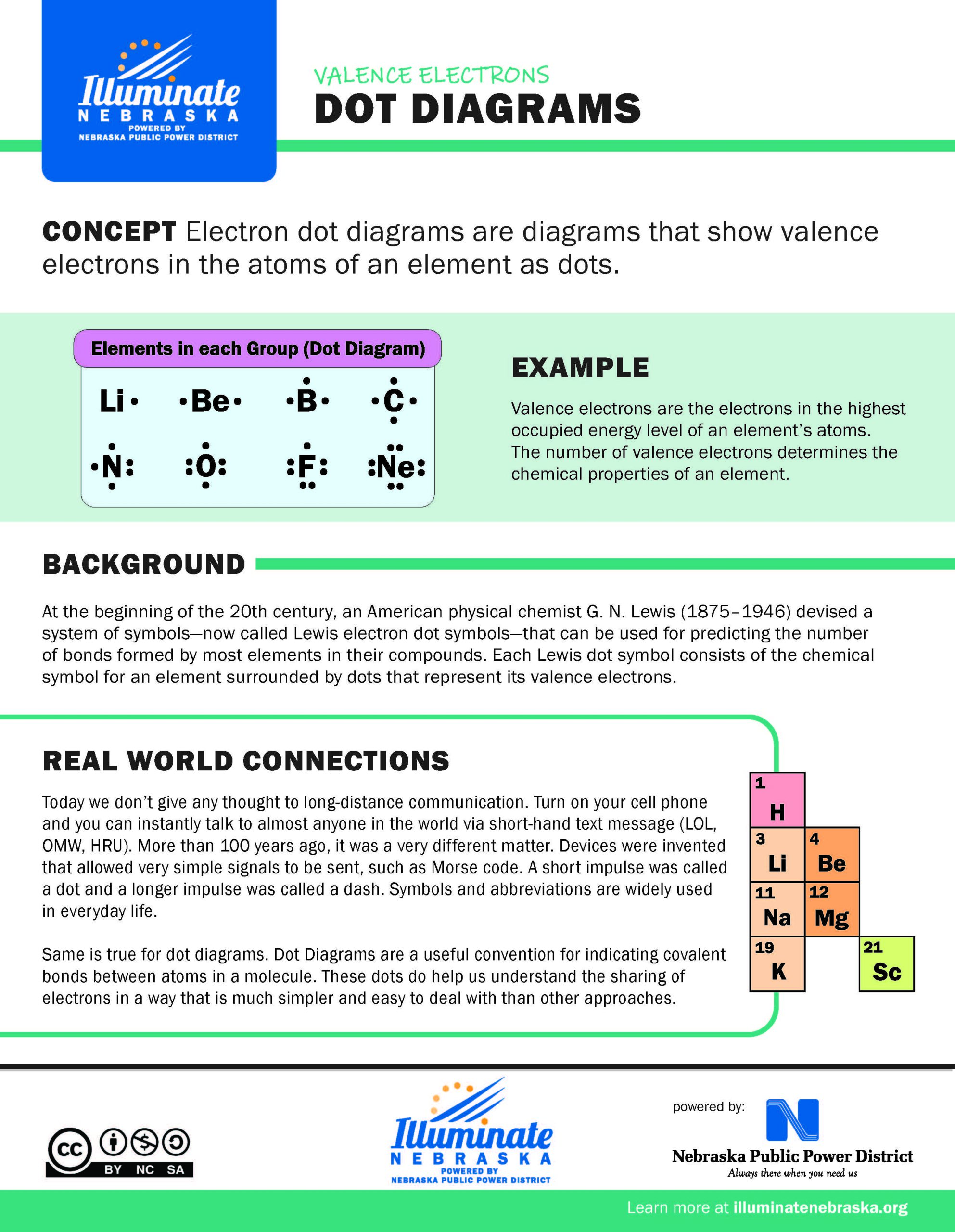
Dot Diagrams
Electron dot diagrams are diagrams that show valence
electrons in the atoms of an element as dots.